by
Ralph C. Merkle,
Xerox PARC,
3333 Coyote Hill Road,
Palo Alto, CA 94304
merkle@xerox.com
Copyright 1993 by Xerox Corporation. All Rights Reserved.
This is a revised version of a paper first published in
Chemical Design Automation News, Volume 8, Numbers
9 & 10, September/October 1993, page 1.
Introduction
Manufactured products are made from atoms. The properties of
those products depend on how those atoms are arranged. Viewed from
the molecular level today's macroscopic manufacturing methods are
crude and imprecise. Casting, milling, welding and all the other
traditional manufacturing methods spray atoms about in great
statistical herds. Even lithography (which already lets us put
millions of transistors on a chip no bigger than your fingernail)
is fundamentally statistical and random. Exactly how many dopant
atoms are in a single transistor and exactly where each individual
dopant atom is located is neither specified nor known: if we have
roughly the right number in roughly the right place, we can make a
working transistor. For today, that is good enough.
The exception is chemistry. Large high purity crystals can have
almost every atom in the right place. So, too, can many long
polymers. The structures of proteins with hundreds and even
thousands of amino acids can be specified down to the last atom.
Most dramatically (and fortunately for us!) DNA strands with many
tens of millions of bases can be copied with almost perfect
accuracy. And it seems that almost any small molecule (with
perhaps several dozens of atoms) can be synthesized, if only we
have the skill and patience.
Yet the laws of physics and chemistry in principle permit
arranging and rearranging the elements in so many combinations
and permutations that all of our manufacturing skills and all of
our chemical skills barely suffice to scratch the surface of what
is possible.
The Utility of Diamond
Almost any manufactured product could be improved, often by
several orders of magnitude, if we could precisely control its
structure at the molecular level. We often want our products to be
light and strong. Diamond is light and strong: the
strength-to-weight ratio of diamond is over 50 times that of steel.
Yet we do not today have diamond spars in airplanes nor diamond hulls
for rockets. Today we can't economically make diamond. Even if we
could, simple diamond crystals can shatter. We'd have to modify
the structure to make it tough and shatter proof: perhaps diamond
fibers. While easily done in principle, we can't do this in
practice today.
Great strength and light weight are not the exclusive province of
diamond: graphite can be stronger. And if we consider the many
ways in which carbon atoms can be arranged and rearranged, then
it's obvious that there are a host of other possibilities. Yet all
share a common problem: we can't yet economically make them in the
exact shapes that we want.
Great strength is only one property that we prize highly: when we
make computers we are more concerned by electrical properties.
Here, too, diamond excels. Today's computers are made of
semiconductors, and the semiconductor of choice is silicon. This
is not because silicon is the ideal semiconductor from which to
make computers, but because we know how to make devices from it.
The computer industry has strong opinions about what makes a good
logic device and what makes a good computer[1, 2], and diamond
will let us make better computers than silicon[3].
Diamond has a wider bandgap, hence electrical devices will work at
higher temperatures. It has greater thermal conductivity, so
devices can be more easily cooled. It has a greater breakdown
field, hence devices can be smaller. It has higher electron and
hole mobility which, when combined with higher electric fields,
will result in higher speed. But again, we see no diamond
computers, just as we see no diamond airplanes: we can't
economically manufacture them yet. Large pure crystals of silicon
can be made relatively easily, but large pure crystals of diamond
are scarce. We can etch the silicon surface and add dopants with
a precision measured in tenths of microns, while the
corresponding steps for diamond are more difficult. Not more
difficult in principle: just more difficult today.
Long Range Complex Order
Making computers highlights another problem. It's not enough to
make a pure crystal, it must also have an extremely precise and
complex pattern of impurities. The exact location of the dopant
atoms in the semiconductor lattice controls how devices function
and where signals can propagate. Local order is crucial to make
each device work, but long range complex order is crucial to make
the computer as a whole work. While we can make some things today
that are highly precise and have simple long range order (e.g.,
crystals), it is the requirement for complex long range order that
prevents us from making computers of the kind we'd like to make.
While it's plausible we could make high density memory from
crystals and perhaps some types of cellular automatons, we
couldn't make anything that resembled the computers on the market
today. Today's high speed semiconductor-based digital computers
(like the 80486 or the Pentium) have millions of logic elements
wired together in complex and highly idiosyncratic patterns.
This is well beyond the capabilities of crystal growth or
bio-polymer synthesis. It will require a fundamentally new
manufacturing technology:
molecular manufacturing.
A Gap
Today, there is a gap in our synthetic abilities: we can make
complex mechanical machinery and electronic devices (including
computers, which have millions of transistors), but we can't make
such devices with the precision with which the chemist can
synthesize a crystal, a bio-polymer, or a relatively small
molecule. With chemistry we can make precise molecular structures
and compounds, but we haven't been able to scale up that success
to molecular computers (and other macroscopic products as precise
as molecules).
Molecular manufacturing
will, by definition, let us economically
manufacture almost any specified structure that is consistent
with the laws of chemistry and physics. To simplify the problem
somewhat we can narrow our focus to structures that resemble
diamond in a broad sense: the diamondoid structures as defined by
Drexler[4]. This class includes (among other things) diamond
crystals of arbitrary shape but with stably terminated surfaces
(e.g., hydrogenated (111) or the like) and with impurities at
precise locations in the diamond lattice (e.g., substitutional
boron). Our objective is to manufacture particular diamondoid
structures once the location and type of every atom has been
specified by design.
The Interest of the Computer Industry
The attraction of molecular manufacturing for the computer industry
should be clear. It should let us make computers at a manufacturing
cost of less than a dollar per pound, operating at frequencies of
tens of gigahertz or more, with linear dimensions for a single
device of roughly 10 nanometers, high reliability, and energy
dissipation (using conventional methods) of roughly 10^-18 joules
per logic operation. If we make
thermodynamically reversible computers
(which the author and others have recently shown can be
made from conventional electronic devices, e.g., CMOS)[5,6,7,8]
then the
energy dissipation per logic operation can be reduced to well below
kT at T = 300 Kelvins (well below 10^-21 joules).
The computer industry is spending billions of dollars to make
better computers. It is widely acknowledged within the industry
that lithography is approaching its limits. Articles like The
Future of the Transistor[1], Miniaturization of
Electronics and its Limits[9] and Outlook for VLSI: Will
the Balloon Burst?[10] quite clearly show that conventional
lithography will run out of steam (in perhaps a decade, though
there is less agreement about the exact time frame). There is
already interest in molecular logic devices[11] and that interest
will increase sharply as improvements in conventional
manufacturing methods become increasingly difficult. However, any
new proposal for manufacturing molecular computers will be
weighed against (at least) the criteria mentioned above. If it
cannot easily beat conventional methods after they have been
pushed to their uttermost limits, then it will be rejected. The
computer industry will soon be pouring vast sums into research
aimed at molecular computing, but the great bulk of funding will
go towards well thought out proposals that offer a realistic
possibility of substantially exceeding the performance of the
ultimately evolved silicon VLSI technology that we expect to
develop over the next decade. If you can't beat tomorrow's
mainstream computers, you might as well not try.
The Problem
For this and many other reasons the class of diamondoid structures
is a reasonable one to consider. The problem of building a
diamondoid electronic computer captures many of the fundamental
issues in
molecular manufacturing,
and poses clearly the issue of
building large structures that cannot be made by regular
repetition of some substructure (e.g., the unit cell of a crystal
or the monomeric unit in a bio-polymer).
This brings us to a core issue in molecular manufacturing: how do
we synthesize such things?
Today, we can synthesize diamond at low pressure and low
temperature by using
CVD (Chemical Vapor Deposition) methods[12,13].
Diamond CVD growth involves highly
reactive species (radicals, carbenes, etc.) in a gas over the
growing diamond surface that bombard and react with that surface
at random. Because reaction sites are random, growth of many
defect structures occurs (dislocations, etc.) as well as the
desired perfect diamond structure.
Two fundamental mechanisms in the growth process include (1)
abstraction of hydrogens from the diamond surface leaving behind
reactive sites (dangling bonds, radicals) and (2) interaction of
carbon species (both reactive (CH2, CH3, etc.) as well as
relatively unreactive species (C2H2)) with the surface, thus
depositing carbon.
If we are to synthesize diamondoid structures it is plausible that
we begin our search for the basic reaction steps involved in this
synthesis by looking at existing reactions that occur in the
CVD
growth of diamond. The use of a reactive gas in the synthesis
process, however, would seem to defeat any hope of making
precisely patterned diamondoid structures, for the gas will
interact with the growing surface at random.
Positional Control is Fundamental
Here, we introduce the fundamental concept of
molecular manufacturing:
positional control over the site of reactions. To
take a specific example we consider site specific hydrogen
abstraction from the diamond (111) surface. The ability to remove
specific hydrogen atoms from the surface of the diamondoid work
piece under construction is likely to be a fundamental unit
operation in any attempt to make atomically precise diamondoid
structures.
Hydrogen abstraction during CVD diamond growth typically involves
a radical reaction between atomic H from the gas with H bonded to
carbon on the surface producing H2. It is unclear how to make
this process site specific. However, there are other structures
with a high affinity for hydrogen which offer greater
possibilities for positional control. In particular, the
propynyl radical C3H3 (figure 1) has a great affinity for
hydrogen. Further, this radical has the very useful property that
it has two ends: one end is a highly reactive radical while the
other end is a stable sp3 carbon. Thus, we could synthesize a
larger molecule with the propynyl radical at its end. The larger
molecule would be held at the tip of a positional device. The
positional device would provide control over the orientation and
position of this hydrogen abstraction tool (e.g., a six degrees of
freedom manipulator) and thus control the site of abstraction by
controlling the position of the tool.
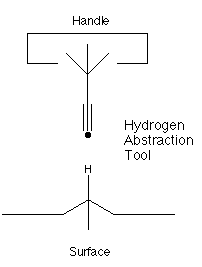
Figure 1. A site specific hydrogen abstraction tool.
Ab initio quantum chemical analysis of the abstraction of hydrogen
from isobutane using an ethynyl abstraction tool supports the idea
that the barrier to this reaction is zero[14]. The reaction will
proceed rapidly and, because of the large exothermicity,
irreversibly. Calculated barriers for abstraction from several
other molecules were also small, suggesting that this hydrogen
abstraction tool could be used to abstract hydrogen from a wide
range of different molecules.
Molecular dynamics simulations[14b] provide
evidence that the abstraction reaction will select the correct
hydrogen atom in the face of thermal noise at room temperature,
as well as providing further support for the basic mechanism.
The site specific abstraction of hydrogen
illustrates the core concept in molecular manufacturing:
selecting the reaction site by controlling the position and
orientation of the reactants. The (relatively stiff) diamondoid
workpiece is held in place, while a tool (in our example, a
hydrogen abstraction tool) is positioned using a rather
conventional (if also rather small) robotic arm[15]. The ideas of
using tools, controlling the position of those tools with a
general purpose manipulator, and building complex structures by
putting together components using those positionally controlled
tools are rather common and even mundane at the macroscopic level.
At the molecular level, they are new and almost shocking: yet it
is simply mapping onto the molecular world the concepts and ideas
that have proven so useful and powerful in macroscopic
manufacturing. By adding positional control we should be able to
develop a method of molecular manufacturing which combines the
best features of both conventional macroscopic manufacturing and
chemical synthesis.
Other Molecular Tools
If we are to grow diamond, we must also have carbon deposition
tools. Drexler has suggested the use of positionally controlled
carbenes (figure 2) and alkynes (figure 3) and proposed reaction
pathways and surface structures where these tools would apply[4].
In both cases, the tools are positioned at a precise point on the
growing diamondoid structure and are used to deposit one or more
carbon atoms at a desired location. These deposition reactions
parallel proposals in the CVD literature except for the addition
of positional control (e.g., at least one portion of the moiety
must be part of an extended "handle" which can be held by a
positional device). These are only two examples from the wide
range of tools that are capable of depositing carbon on a surface.
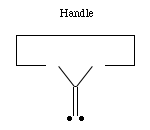
Figure 2. A positionally controlled carbene
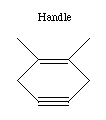
Figure 3. A positional controlled strained cycloalkyne
The broad range of possible tools coupled with the great power of
ab initio computational chemistry should let us define and verify
a complete set of molecular tools capable of synthesizing
essentially any diamondoid structure.
The work by Musgrave et. al.[14]
and Sinnott et. al. [14b] are first steps toward this
objective. Modern ab initio methods can produce results that are
sufficiently accurate for this type of analysis[16, 17].
Further research in this area is feasible and should be pursued.
The Context of Tool Use
For such tools to be usable in a system context we must satisfy
certain constraints. First and foremost, we must have a device
capable of positioning the tool to within something like an atomic
diameter. On the diamond (111) surface, the distance between
adjacent hydrogens is about 2.5 Angstroms. Thus, positional
accuracy of 1 to 2 angstroms for the hydrogen abstraction tool is
required to prevent abstraction of the wrong hydrogen. Second,
because the tools can be highly reactive, we require an inert
environment. A simple inert environment is vacuum. Compressed
helium or some other inert gas would also work. Third, because it
is the relative tool-workpiece position that must be controlled,
the workpiece under construction must be relatively rigid (e.g.,
not subject to vibrational motions that would exceed about an
angstrom). Fourth and last, we must have some way of generating
the sometimes highly reactive tools (e.g., we need to define a
precursor to the hydrogen abstraction tool, as well as precursors
to the other tools).
In some sense, the analysis that we will now pursue is similar in
type to retrosynthetic analysis[18]. We start with the final
product that we wish to build (a macroscopic diamondoid computer,
for example), and then consider the possible predecessor
structures which would yield the final product in one step. Then
we consider the predecessor structures to those structures, and
so on. We extend retrosynthetic analysis beyond its traditional
bounds, but the general concept remains the same: given the
finished product we deduce the possible ways in which it could
have been constructed. This approach has been called "backwards
chaining" by Drexler[4].
Positional Devices and Molecular "Arms"
Work with SPMs (Scanning Probe Microscopes)[19] clearly show
that it is possible to achieve positional accuracies of a small
fraction of an angstrom. Small (~0.1 microns) diamondoid "arms"
or positional devices with similar positional accuracy are in
principle quite feasible. The field of robotics provides a broad
range of designs for positional devices[15] which are largely
scale independent. Shrinking these designs to submicron size is
conceptually straightforwards. A factor of crucial importance in
the design of molecular-scale positional devices is the accuracy
with which the tip can be positioned, particularly in the face of
thermal noise. While
atomically precise bearings and joints will
not suffer from chatter, backlash, wear, tooth-to-tooth errors
and other sources of inaccuracy caused by imprecise
manufacturing[4, 20], they will still suffer from positional
errors caused by thermal noise. To control this source of error,
it is essential that the robotic arm be very stiff, and so the use
of stiff materials is desirable. The Young's modulus for diamond
is about 10^12 Pascals (very stiff), and back-of-the-envelope
calculations show that a hollow cylinder of such material that is
perhaps 100 nanometers long and 30 nanometers in diameter should
have a positional accuracy at the tip, in the face of thermal
noise at room temperature, of a small fraction of an atomic
diameter. A more detailed design and analysis of a jointed
tubular robotic arm taking into account the bending and rocking
motions of joints in the arm further supports this conclusion[4].
Alternatives
to the simple robotic arm are available which might
be more attractive.
Other Requirements for Tool Use
Creating an inert environment also presents no fundamental
problems: high quality vacuums are common in laboratories today.
If our objective is to have a very small very high quality vacuum,
then a relatively thin wall of diamondoid material could be used
as a barrier to keep a volume which was a modest fraction of a
cubic micron free of any contaminants. If the volume were
initially constructed free of contaminants then such a barrier
would keep the inside free of any contaminants with high
probability.
Because we are building diamondoid structures, they will be very
stiff. As a consequence, it is relatively easy to meet the
requirement that the objects that are being manufactured must
themselves be stiff.
Finally, generation of "activated" tools from relatively stable
precursors can be done by a variety of methods. Because we are
assuming an environment in which we have positional control we can
use particularly simple precursors. We illustrate this by
considering a precursor to the hydrogen abstraction tool (see
figure 4). This precursor has two handles, and X is chosen so
that the X-C bond is weaker than the C-C bond. X might be
Si or Ge.
If we pull on the two handles with sufficient force,
something will break. Because the X-C bond was deliberately
selected to be weaker than the other bonds in the structure, it
will break. This gives us the activated hydrogen abstraction
tool.
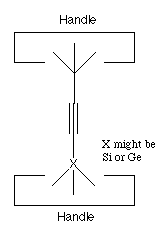
Figure 4. A possible precursor to the hydrogen abstraction tool of
figure 1.
A related question is: how can we get the hydrogen off the tip of
the abstraction tool? A simple answer is: don't. Throw the tool
away after one use. In a system design using this approach, it
would be necessary to provide a continuous stream of precursors.
These would be activated, used once, and then discarded. A more
elegant approach would be to remove the hydrogen from the tip and
recycle the tool, as discussed by
Drexler[4] and
Musgrave et. al.[14].
More generally the activation of relatively stable precursors can
be done by using any of several forms of energy: mechanical,
optical, chemical or other. While the use of mechanical means to
provide the activation energy for chemical reactions is
relatively novel, in an environment where positional control is
already available it is quite natural.
Selective Transport Across a Barrier
Having introduced a diamondoid barrier to keep unwanted
contaminants out (much as the bacterial wall allows bacteria to
maintain an appropriate internal environment in the face of a
fluctuating external environment) we must now solve the problem
of getting desired raw materials through the barrier. We might,
for example, wish to transport the hydrogen abstraction tool
precursor across the diamondoid barrier. After use we will also
need to eject the spent tool. Several ways to solve this problem
are feasible. A proposal by Drexler is to use a rotor embedded in
the diamondoid wall which moves binding sites from the outside of
the wall to the inside of the wall (figure 5 [4, page 378]). By
modulating the affinity of the binding site so that it will have
high affinity for the desired molecule outside the barrier and low
affinity inside the barrier, efficient transport across the
barrier can be achieved. The desired molecule will bind to the
binding site when it is outside the barrier, the binding site will
be rotated to the inside of the barrier and the binding affinity
reduced (in the illustrated proposal by mechanically pushing a
rod into the binding site, thus physically precluding occupancy),
and the molecule will be released on the inside of the barrier.
The result is to increase the concentration of the desired
molecule. A few stages of such a filtration system can achieve
extremely high purities. The final stage, rather than ejecting
the molecule into a liquid, would deliver the molecule into the
inert internal environment in a well defined orientation where it
could be further processed. One simple method of further
processing would be for the oriented molecule to be directly
transferred to the tip of the positional device.
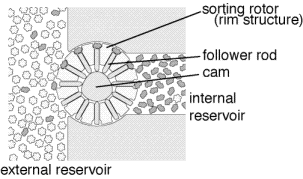
.
Control Signals
Finally, we will need a source of control signals for our
molecular arm. One general approach would be to use a molecular
computer. We will not consider a particular design for a
molecular computer here, it is sufficient to note that many
proposals for molecular computation have been considered in the
literature and it is generally expected that some type of very
small computational device will be feasible in a few decades[4,
11].
Summary
This completes our (all too brief) outline of a small device able
to manufacture a broad class of diamondoid materials. Basically,
the design is driven by the desire to provide the environment
needed to synthesize diamond and diamondoid materials using the
kinds of reactions that occur naturally during CVD growth of
diamond. The device is itself made from diamondoid materials,
which means that one such device can manufacture a second such
device. This ability to self replicate is crucial in achieving
low manufacturing costs. As the reader might appreciate, the
design and construction of one such general purpose device might
well prove to be a time consuming and expensive undertaking.
This cost cannot be justified unless the resulting device has
great value. If the device can self replicate then the successful
design and development of one such device can be used to build an
entirely new manufacturing technology. The manufacturing costs
for the second, third, fourth, .... 10^10.... etc. devices will
consist largely of the raw materials and energy costs. Thus, a
very large R&D cost can (if necessary) be justified.
A number of technical issues involved in self replicating systems
are discussed in
Self Replicating Systems and Molecular Manufacturing
[21] while some of the obvious safety issues are
discussed in The Risks of Nanotechnology[22].
Bricks Without Vacuum
The major driving force in the previous design was the desire to
approach fundamental limits (strength, stiffness, thermal
conductivity, electrical characteristics, etc.) in the
manufactured products. This in turn implied the use of diamondoid
materials, which, when coupled with the known chemistry of CVD
diamond synthesis resulted in a high vacuum system with highly
reactive intermediates.
Relaxing the materials requirements gives us a much wider
range of possible structures. In particular, we can consider what
is sometimes called "brick" or "building block" based
nanotechnology. In this approach we first design a set of
molecular building blocks, and then assemble the building blocks
by the use of positional control. The ribosome can be viewed as
the prototype for this approach. The building blocks are amino
acids, and they are linked together by the ribosome to form
proteins. Our approach differs in two principle respects: first,
we add positional and orientational control over the building
blocks in three dimensions, while the ribosome can only build
structures that are fundamentally one dimensional (relying on
linear structures that spontaneously fold into a particular shape
to achieve a degree of control in three dimensions). Second,
rather than using relatively floppy polymers, we prefer
relatively rigid bricks that can be bonded to each other in a
stiff three-dimensional framework.
In general, molecular structures built of bricks will be (a)
larger (b) weaker (c) less stiff (d) have poorer thermal
conductivity (e) have poorer electrical properties (f) etc. etc.
etc. The sacrifice made in materials properties is significant.
On the other hand, many bricks can be assembled in more
conventional environments (solution), and so we can eliminate the
need for vacuum. This greatly simplifies the system. Indeed,
with brick-based nanotechnology one can relatively easily
envision the synthesis of a set of bricks that can, with the
addition of positional control, be assembled into a wide range of
structures with the stiffness of (say) wood.
Molecular Manipulators
A conceptually simple and relatively near-term way to achieve
some degree of positional control would be to use conventional
SPMs. The tips used in current SPM's are usually quite crude, and
even when it is possible to make a very fine tip the range of
possible structures is very limited (tungsten or some other simple
material is generally all that's available). The design of tips
for the SPM that incorporate individual molecules specifically
synthesized for the purpose is a likely next step, and one that
seems essential if we are to make progress in using SPM's to guide
chemical reactions in a selective way.
Such a "molecular manipulator" should be within reach of today's
experimental technology. While the Young's modulus of the things
it could make would be substantially inferior to that of diamond,
this can be compensated by making them bigger. Scaling laws are
such that increasing the size of an object by a factor of 10 also
increases its stiffness by a factor of 10, and so reduces the
positional inaccuracy from thermal vibration by a factor of 10.
If we wish to build a molecular "arm" out of bricks, then to
achieve the same positional accuracy as with a diamondoid arm we
will have to make the arm bigger -- perhaps several tenths of a
micron or more -- but the basic design concepts that were
discussed previously for use with a diamondoid arm still hold. We
can still position the individual bricks accurately both in
position and orientation, we can still build larger structures by
putting together many bricks, we can still control the synthesis
process by positional means, we can still make a positional device
from bricks, etc.
Why pursue such an approach when it can only make relatively
inferior materials? There are three primary reasons: (1) it's
easier to do (2) it could still make many things that are very
valuable by today's standards and (3) such systems could be used to
make better systems (e.g., diamondoid systems).
As the reader will appreciate, there are many possible candidates for bricks.
Many researchers are already considering the design of molecular building blocks[23],
although in most cases they do not consider positional control. Krummenacker
discusses several of the issues surrounding the design of molecular bricks
intended for assembly via positional control[24]. Adding positional control
makes the synthesis of a broad range of molecular structures feasible, but at
the same time requires the design and synthesis of a mechanism able to provide
such control. The obvious first step is to provide positional control using
relatively modest extensions to today's SPMs. The most significant addition
is the inclusion of a molecular tip, for today's SPMs typically have a limited
range of possible tip structures and are often imprecise at the molecular scale.
Conclusion
The long term goal of molecular manufacturing is to build exactly
what we want at low cost. Many if not most of the things that
we'll want to build are complex (like a molecular Cray computer),
and seem difficult if not impossible to synthesize with currently
available methods. Adding programmed positional control to the
existing methods used in synthesis should let us make a truly
broad range of macroscopic molecular structures. To add this kind
of positional control, however, requires that we design and build
what amount to very small robotic manipulators. If we are to make
anything of any significant size with this approach, we'll need
mole quantities of these manipulators. Fortunately, any truly
general purpose manufacturing device should be able to
manufacture another general purpose manufacturing device, which
lets us build large numbers of such devices at low cost. This
general approach, used by trees for a very long time, should let
us develop a low cost general purpose molecular manufacturing
technology.
While we have focused in this article on diamondoid structures and molecular
computers based on semiconductors such as diamond, it will probably be easier
to first make systems that rely on materials that are simpler to synthesize
but whose material properties are not as good as diamond. The general concept
of positional control, however, still applies. A future article will discuss
in greater detail the design of such simpler systems, and how they can form
a stepping stone to mature molecular manufacturing.
Many challenges must be met and it will be many years before we
develop molecular manufacturing; but the goal is worthwhile,
achievable, and offers great rewards both financial and
scientific.
References
1) Keyes, R.W., 1993, Scientific American Vol 268 No. 6 page
70-78. The Future of the Transistor.
2) Keyes, R.W., (1991) Contemporary Physics, Volume 32, No. 6,
pages 403-419. Limits and Challenges in Electronics.
3) Geis, M.W., and Angus, J.C., (1992) Scientific American,
October page 84. Diamond Film Semiconductors.
4) Drexler, K.E., (1992)
Nanosystems: Molecular Machinery, Manufacturing,
and Computation, Wiley.
5) Koller, JG and Athas, WC, (1992)Physics of Computation
Workshop, October 2-4, Dallas Texas. Adiabatic Switching, Low
Energy Computing, and the Physics of Storing and Erasing
Information
6) Merkle, RC, Nanotechnology (1993) 4, pp 21-40.
Reversible Electronic Logic Using Switches.
7) Hall, JS, (1992) Physics of Computation Workshop, Dallas Texas,
Oct 2-4. An Electroid Switching Model for Reversible Computer
Architectures.
8) Bennett, CH and Landauer, R (1985) Scientific American, July,
pp 48-56. The Fundamental Physical Limits of Computation.
9) Keyes, R.W., (1988) IBM J. of Res. Development, Vol. 32, No.
1, January pages 24-28. Miniaturization of Electronics and
its Limits.
10) Gordon Moore, 1993, Bulletin of the American Physical
Society, Vol 38. No. 1, page 298, session G2. Outlook for
VLSI: Will the Balloon Burst?
11) Carter, L., Siatkowski, R.E., and Wohltjen, H; 1988,
Molecular Electronic Devices, North-Holland.
12) Angus, JC (1989) pp 1-13 in Proceedings of the First
International Symposium on Diamond and Diamond-Like Films
edited by J.P. Dismukes et. al., The Electrochemical Society.
History and Current Status of Diamond Growth at Metastable
Conditions
13) Celii, FG and Butler, JE, (1991)Annu Rev. Phys. Chem. 42 pp.
643-684. Diamond Chemical Vapor Deposition
14) Musgrave CB, Perry, JK, Merkle, RC, and Goddard, WA,
Nanotechnology (1991) 2 187-195 Theoretical studies of a
hydrogen abstraction tool for nanotechnology
14b) Sinnott SB, Colton RJ, White CT, and Brenner DW,
Surface Science 316 (1994) pages L1055 to L1060
Surface patterning by atomically-controlled chemical forces:
molecular dynamics simulations
15) Klafter, RD, Chmielewski, TA, and Negin, M, (1989)
Robotic Engineering: an Integrated Approach Prentice Hall.
16) Goddard, W. A, (1985) Science, Feb. 22, page 917.
Theoretical Chemistry Comes Alive: Full Partner With
Experiment.
17) Schaefer, HF (1986) Science, Vol 231 page 1100.
Methylene: A Paradigm for Computational Quantum Chemistry
18) Corey, E.J., and Cheng, X-M, (1989)The Logic of Chemical
Synthesis, Wiley.
19) Eigler, DM and Schweizer, EK, (1990), Nature 344 April 5, pp.
524-526. Positioning single atoms with a scanning tunnelling
microscope.
20) Merkle, RC, (1993) Nanotechnology 4(2) 86-90,
A Proof About Molecular Bearings.
21) Merkle, RC (1992) Journal of the British Interplanetary
Society, Vol 45, pp. 407-413.
Self Replicating Systems and Molecular Manufacturing.
22) Merkle, RC (1992) pp 287-294 in Nanotechnology, Research
and Perspectives, by BC Crandall, MIT press.
23) Amato, I., (1993) Science Vol. 260, May 7, pp 753-755.
Designer Solids: Haute Couture in Chemistry.
24) Krummenacker, M. (1994), Steps towards molecular manufacturing, Chemical Design Automation
News, volume 9, January, pages 1 and 29-39.
 This
page is part of the
nanotechnology web site.
This
page is part of the
nanotechnology web site.






 This
page is part of the
nanotechnology web site.
This
page is part of the
nanotechnology web site.